Acetone Polar or Nonpolar Solvent
It is common to try three to six solvent systems for the first round of. Also any potential hydrogen-bond acceptor will tend to shift the water signal down-field.

Is C3h6o Acetone Polar Or Non Polar Youtube
Polar solvents can be used to dissolve inorganic or ionic compounds such as salts.

. Add 100 mL of a chosen nonpolar solvent to another beaker. ScCO 2 is a promising green solvent because of its negligible toxicity and high solubility window for different kind of solutes. Sodium chloride has a NaCl molecule which breaks into Na and Cl- ions when dissolved in water.
Why is acetone a better solvent than water. R - COOH Carboxylic acids Ethanoic acid H - OH Water Water Nonpolar Polar Increasing Polarity. The conductivity of a solution depends on the solvation of its ions.
They found that the dissolution rate decreases with increasing solvent size indicating that dissolution rate is limited by the rate of which solvent molecules penetrate. The polarity differentiation is done based on the dielectric constant of the solvents. Many nonpolar solvents fall into the category of volatile organic compounds VOCs and are quite dangerous.
And nonpolar gases such as ammonia ethanol and acetone at room temperature mostly because of their metallic core channels and surface functional groups that result in strong adsorption energies. The structure of the fatty acids determines whether or not the fat is. Secondly since it is miscible acetone is a strong solvent ensuring it has the potential to blend in all amounts with water.
It should be noted that the latter is quite temperature-dependent videinfra. The most common three types of solvents in organic chemistry are apolar polar aprotic and polar protic. Water-free synthesis of Ti 3 C 2 T x MXene in ammonium dihydrogen fluoride NH 4 HF 2 dissolved in polar organic solvents eg propylene.
These are solvents having a dielectric constant of more than 15. Polar solutes will dissolve better in polar solvents and nonpolar solutes will dissolve better in nonpolar solvents. Water as a solvent dissolves sodium chloride by breaking into ions.
That is solutes typically will dissolve best in solvents that have the most molecular similarities. It is also produced as a by-product in many large scale production of compounds. Acetic acid is a polar protic solvent with a dielectric constant of 62 in its liquid form.
Additionally solutes will be more soluble if the molecules in the solute are smaller than the ones in the solvent. A solvent from the Latin solvō loosen untie solve is a substance that dissolves a solute resulting in a solutionA solvent is usually a liquid but can also be a solid a gas or a supercritical fluidWater is a solvent for polar molecules and the most common solvent used by living things. SOLVENT MISCIBILITY TABLE.
Acetic acid undergoes decomposition when heated above 440C to yield either methane and carbon dioxide or water and ethanone given by the equations. Waxes steroids phospholipids and fats are the most common types of lipid groups. Factors Affecting R f Values 1.
08 then add more nonpolar solvent or switch to an even less polar combination such as pentaneether. Many polymeric materials having chain-like structures similar to polyethylene are known. Polymers formed by a straightforward linking together of monomer units with no loss or gain of material are called addition polymers or chain-growth polymersA listing of some important addition polymers and their monomer precursors is presented in the following table.
Nonpolar substances are not likely to dissolve to a significant degree in polar solvents. In contrast in eg. Polar solvents are often found to have a high dielectric constant although other solvent scales are also used to classify solvent polarity.
This is particularly true for nonpolar solvents. Acetic acid undergoes nearly all carboxylic acid reactions. DMSO the water is already strongly.
R - COOR Esters Ethyl acetate R - CO - R Aldehydes Acetone methyl ethyl and ketones ketone R - NH 2 Amines Pyridine triethylamine R - OH Alcohols Methanol ethanol. Add a nonpolar solvent to a new beaker. The analytes the solvent front and the point where the mixture AB was administered are all measured relative to each other.
Solvent Impacts Retention Factors. For binary mixtures of acetoneisopropanol a transition from swelling to dissolution occurred near acetone volume fractions of 04505. Because the solvent transports the chemical along the plate the solvent utilised has a significant impact on the chemicals retention factor value.
Nonpolar solvents cannot solvate ions and ions will. For example nonpolar molecular substances are likely to dissolve in hexane a common nonpolar solvent. They can dissolve salts and other ionizable solutes.
Two additional guidelines are derived from these. Fats have glycerol in addition to three fatty acids. Let this beaker sit beside the water beaker.
Nonpolar solvents include things like toluene gasoline and oils. Lipids as a class of compounds are insoluble in water but are soluble in other organic solventsExamples of such solvents include acetone and ether. Enter the email address you signed up with and well email you a reset link.
Shifts of the solvent residual peak2 and the water peak. For example nonpolar molecular substances like hydrocarbons are likely to be insoluble in water. Owing to its ability to dissolve both polar and nonpolar compounds acetone is a strong solvent while other solvents can only dissolve one or the other.
Its high diffusivity and variable density in the supercritical region replaced many conventional and toxic solvents 9It provides a green pathway for the many chemical reactions. Apolar or nonpolar solvents have low dielectric constants which refers to the electric charge between the molecules being evenly distributed. All the ions and proteins in a cell are dissolved in water within the cell.
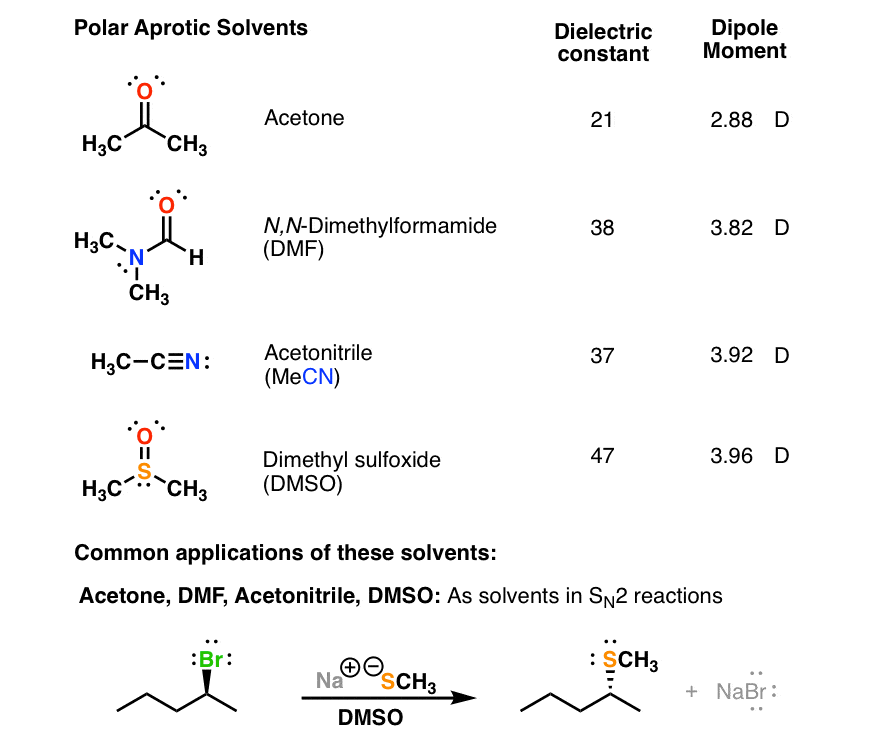
Polar Protic Polar Aprotic Nonpolar All About Solvents
Polar Vs Nonpolar Solvents Identifications And Examples Psiberg
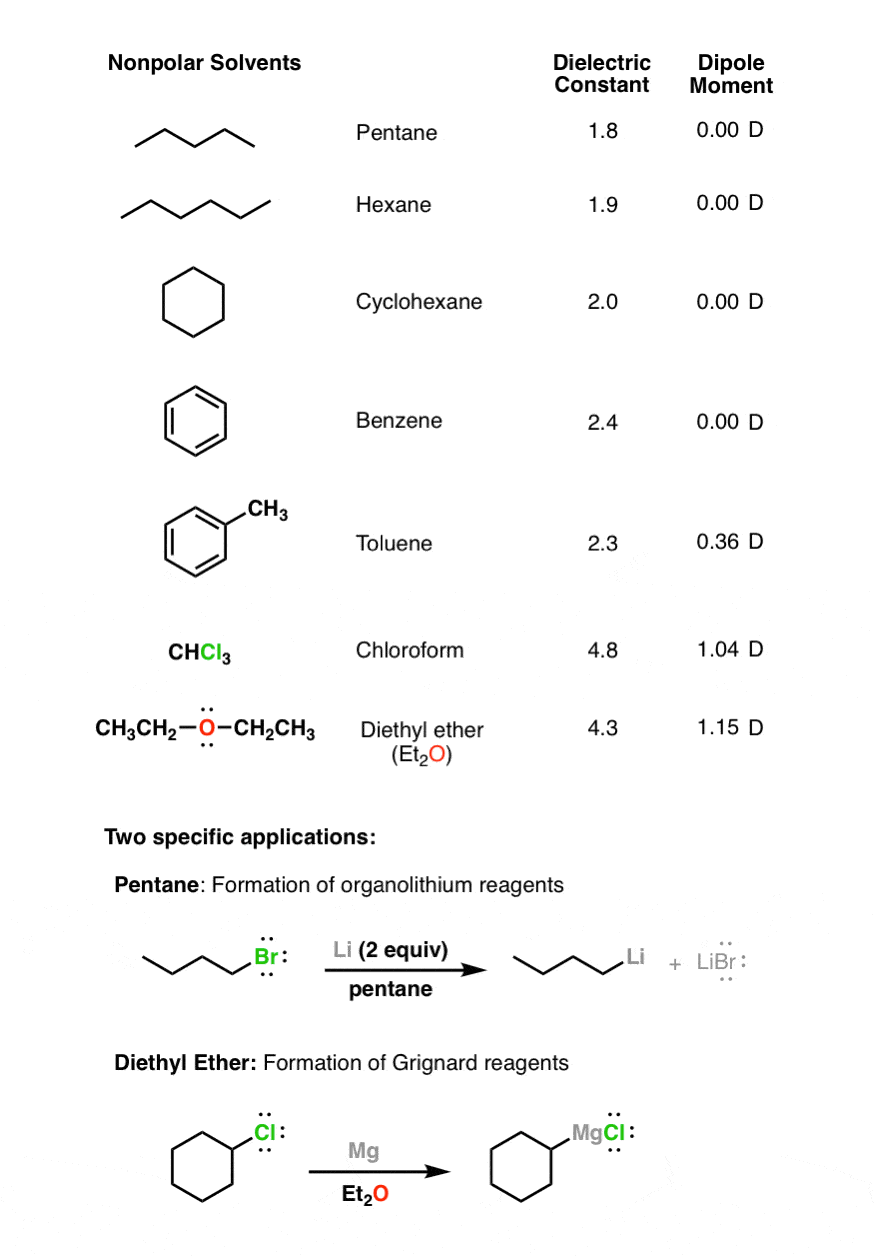
Polar Protic Polar Aprotic Nonpolar All About Solvents

Why Is Acetone A Good Solvent Properties Explanation Video Lesson Transcript Study Com
Comments
Post a Comment